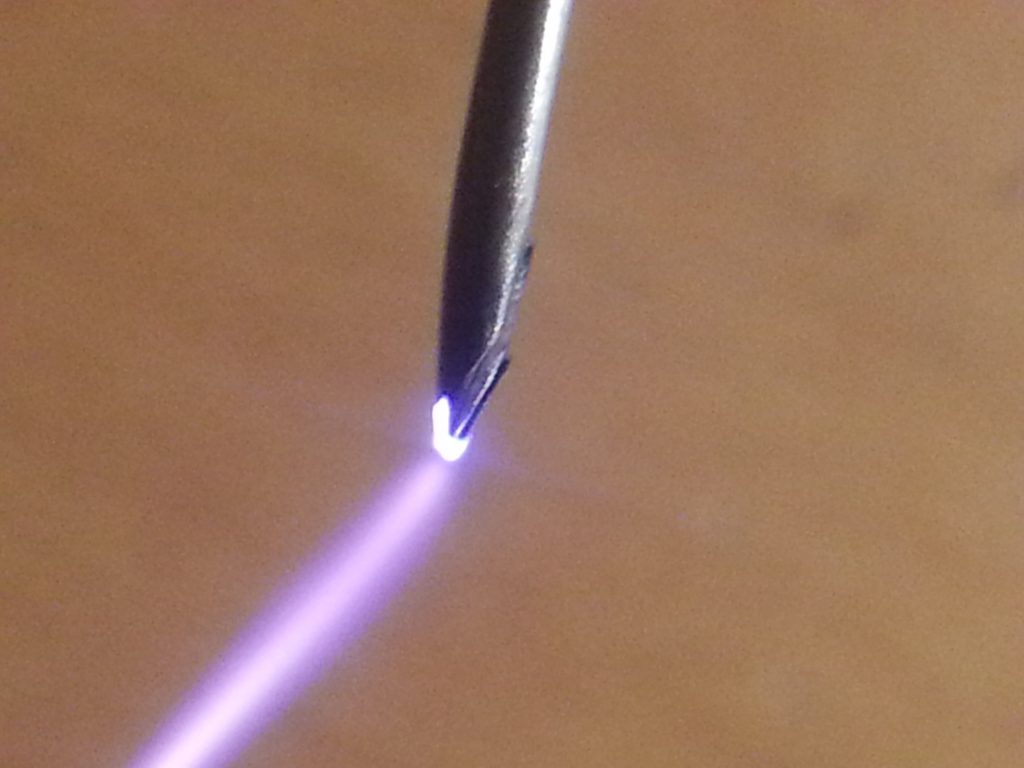
In need of some nitric acid and lacking the required chemicals I found out about the Birkeland–Eyde process.
By creating a high voltage arc in normal air a few chemical reactions occur because of the hot plasma.
Firstly the oxygen and nitrogen react to form nitric oxide.
(N₂ + O₂ –> 2NO)
While cooling it further reacts and oxidizes to form nitrogen dioxide.
(2NO + O₂ –> 2NO₂)
Then when nitrogen dioxide comes into contact with water it reacts to create nitric acid.
(3NO₂ +H₂O –> 2HNO₃ + NO)

My first attempt was rather primitive, creating high voltage from the flyback transformer from an old crt tv,
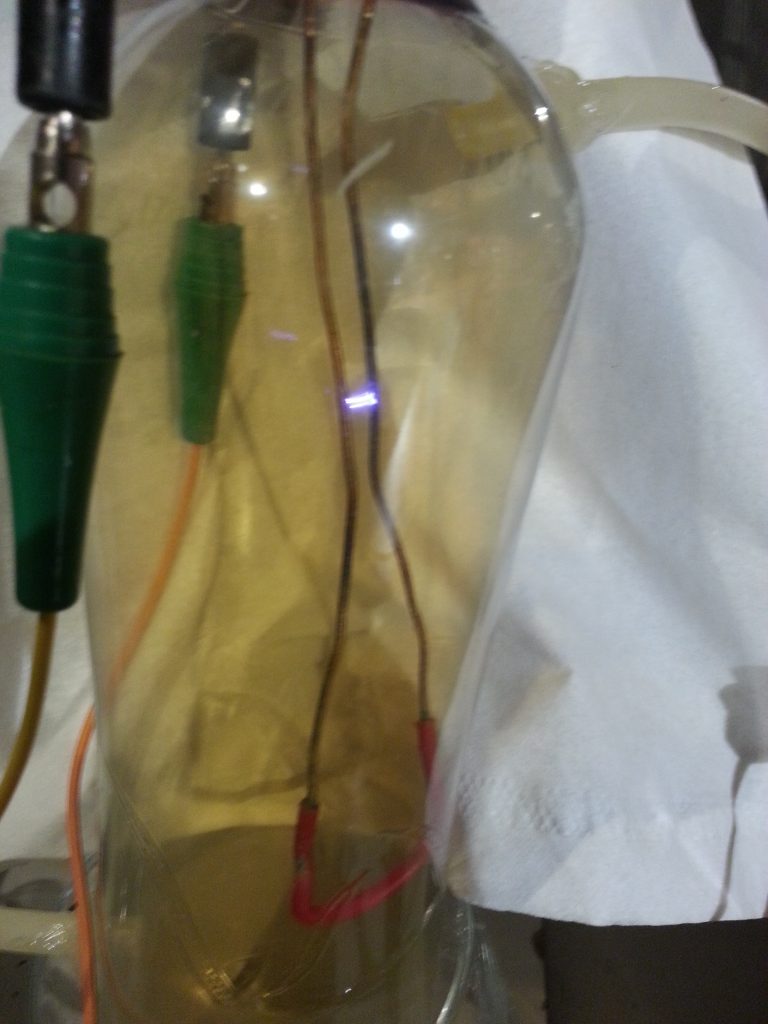
2 electrodes stuck inside a plastic bottle to create a jacobs ladder, some tubes with a syringe to manually pump the air through once some nitrogen dioxide accumulated. and then a tube submerged in water with some cotton to create small bubbles to create the nitric acid.
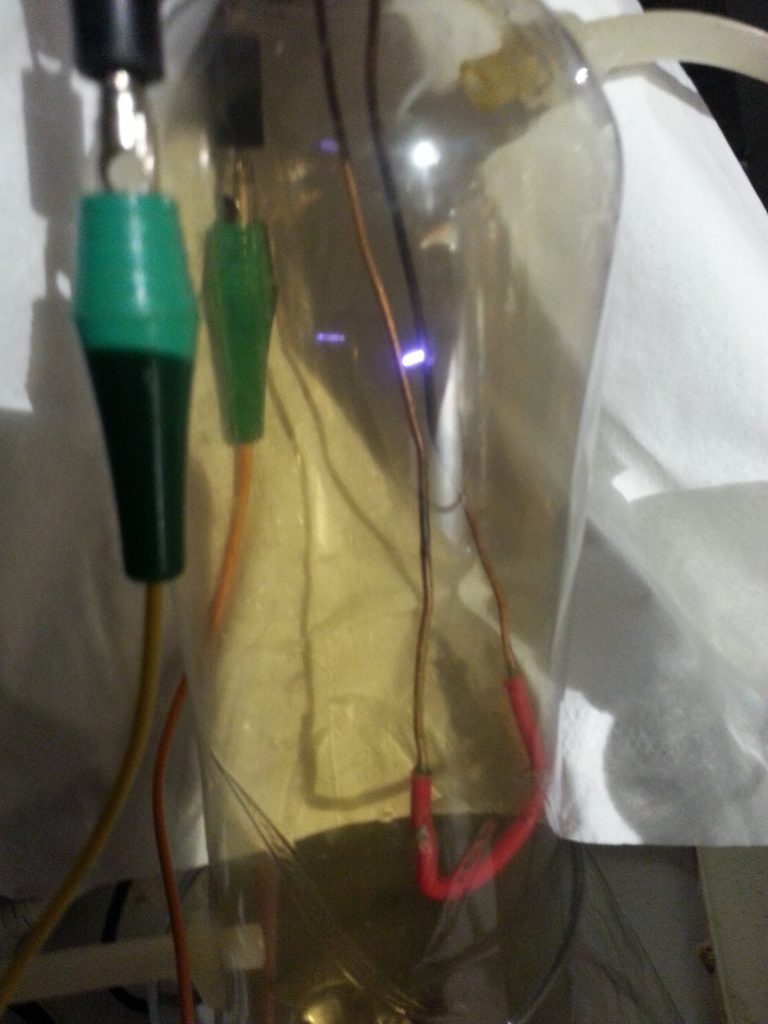
after a few hours I managed the get the PH level pretty low but this was with only a very small amount of water so this setup was good for testing but not for producing any usable amount.

In the end, I produced less than 100ml nitric acid in varying degrees of concentration.
Underneath are all photos relating to the project I could find.
It has been 5 years since I tried this so some photos and details I have lost and forgotten.